Tolerance
BROVANA: No tolerance over 6 months as measured by mean change in FEV1 AUC0-6 from baseline1,2
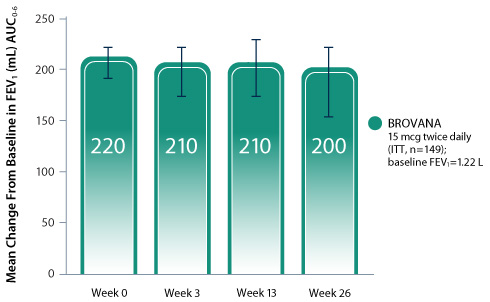
Double-blind, double-dummy, multicenter, randomized, active-controlled, parallel-group safety study that evaluated the long-term safety of nebulized BROVANA 15 mcg twice daily in 149 patients with COPD over 6 months. Formoterol was an active comparator.1,2
- The primary endpoint of this study was to evaluate the overall occurrence of adverse events including COPD exacerbations
- While primarily designed to assess safety, secondary endpoints included FEV1 change and percent change in FEV1 from study baseline and visit predose1,2
BROVANA is indicated for the long-term, twice-daily (morning and evening) maintenance treatment of bronchoconstriction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema. BROVANA is for use by nebulization only.
BROVANA is not indicated for the treatment of acute episodes of bronchospasm, ie, rescue therapy, and does not replace fast-acting rescue inhalers.
BROVANA should not be initiated in patients with acutely deteriorating COPD, which may be a life-threatening condition.
BROVANA should not be used in conjunction with other inhaled, long-acting beta2-agonists.