Duration
BROVANA: 12-hour Bronchodilation
Significant improvement in mean FEV1 change from baseline over the 12-hour dosing interval at Week 121
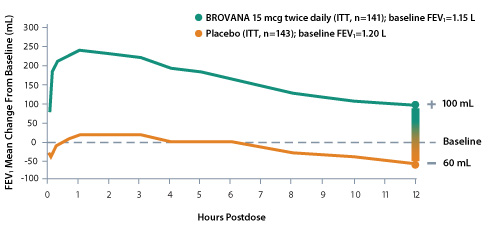
Mean change from baseline in FEV1 at Week 12 in a 12-week, multicenter, randomized, double-blind, placebo-controlled trial to assess the efficacy and tolerability of nebulized BROVANA 15 mcg vs placebo in patients with COPD. Salmeterol was an active comparator, and there was no statistically significant difference between the BROVANA and salmeterol treatment groups for the primary efficacy endpoint.1,2
- In an ad hoc analysis of 212 patients, the majority (56%, n=118) of patients had ≥100 mL improvement in trough FEV1 at Week 12*3
- Overall efficacy was maintained throughout the 12-week study period. While some tolerance to the bronchodilator effect was observed after 6 weeks of dosing (at the end of the dosing interval), it was not accompanied by other clinical manifestations of tolerance1,4
- BROVANA improved morning symptom ratings (assessed nighttime symptoms) from baseline†2,5
- BROVANA increased mean symptom-free nights by 19% from baseline over 12 weeks*†6
- All patients (including those in placebo group) received rescue albuterol and supplemental ipratropium for use as needed throughout trial, except within 6 hours of PFT visit