Symptom Relief
BROVANA: 12% more patients reported improvement in overall COPD symptoms vs placebo*1
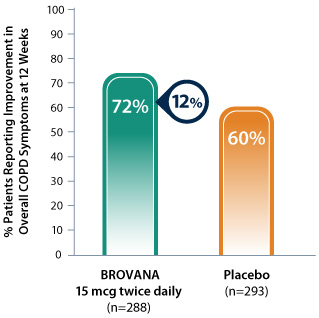
Data from 2 identical 12-week, double-blind, placebo-controlled, randomized, multicenter trials (trials A & B) assessing the efficacy and tolerability of nebulized BROVANA 15 mcg twice daily (n=288) vs placebo (n=293) in patients with COPD.
* Patients rated overall COPD symptoms compared with before study enrollment using the following scale: much better, moderately better, slightly better, the same, slightly worse, moderately worse, much worse.
- All patients (including those in placebo group) received rescue albuterol and supplemental ipratropium for use as needed throughout trial, except within 6 hours of PFT visit
- Patients who reported their overall COPD symptoms as slightly, moderately, or much better were considered to have improvement vs baseline