Dyspnea
BROVANA: Reduced dyspnea — 25% more patients had ≥ 1-unit improvement in TDI at Week 12 vs placebo*1
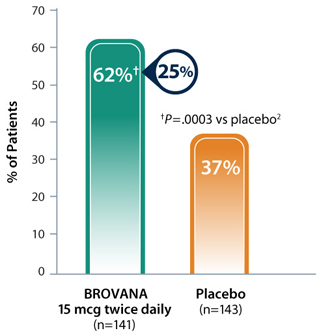
Patient-reported improvements in dyspnea reflected by mean TDI scores at Week 12 in a 12-week, multicenter, randomized, double-blind, placebo-controlled trial to assess the efficacy and tolerability of nebulized BROVANA 15 mcg twice daily vs placebo in patients with COPD.
Albuterol MDI and ipratropium MDI were provided to all patients as rescue and supplemental medications, respectively, for use as needed throughout the trial, except within 6 hours of the PFT visit.1
* TDI is used in conjunction with the Baseline Dyspnea Index (BDI) to assess changes in breathlessness over time. The BDI is determined first, then the TDI scores are taken in subsequent patient visits to determine changes to relative baseline. The TDI score is measured in units, or numbers, from -9 to 9, rounded to the nearest tenth. Values are assigned to specific criteria of each of the 7 grades (change) of 3 components (functional impairment, magnitude of task, and magnitude of effort).
TDI=Transition Dyspnea Index.
- 25% more patients in the BROVANA treatment group experienced a clinically significant (≥1 unit) improvement in TDI compared with the placebo group1
- Mean TDI score for BROVANA was 2.0 units, and for placebo was 1.1 units1
- An improvement in TDI score of ≥ 1 unit is considered to be clinically important3
As with other inhaled beta2-agonists, BROVANA can produce paradoxical bronchospasm that may be life-threatening. If paradoxical bronchospasm occurs, BROVANA should be discontinued immediately and alternative therapy instituted.