Nighttime
BROVANA: More symptom-free nights vs placebo over 12 weeks1
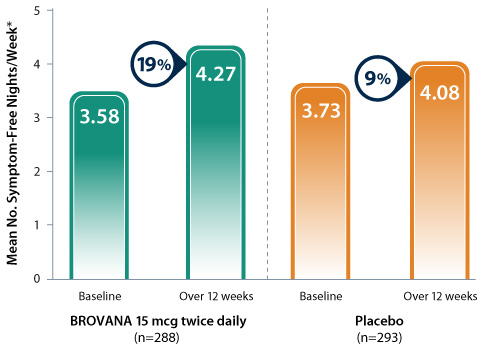
Data from 2 identical 12-week, double-blind, placebo-controlled, randomized, multicenter trials (trials A & B) assessing the efficacy and tolerability of nebulized BROVANA 15 mcg twice daily (n=288) vs placebo (n=293) in patients with COPD.
* Patients completed a COPD questionnaire upon rising in the morning to assess nighttime symptoms. Patients reported the number of times they woke during the night due to lung disease symptoms.
- Patients taking BROVANA reported a 19% increase in mean number of symptom-free nights per week over the 12-week study period compared to baseline
- A symptom-free night was defined as a night in which a patient reported he/she did not awaken due to lung disease symptoms (such as coughing, wheezing, chest tightness, bringing up mucus, shortness of breath)