Exacerbations
BROVANA: Fewer COPD patients reported exacerbations vs placebo1
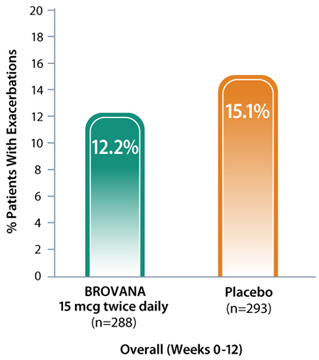
Data from 2 identical 12-week, double-blind, placebo-controlled, randomized, multicenter trials (trials A & B) assessing the efficacy and tolerability of nebulized BROVANA 15 mcg twice daily (n=288) vs placebo (n=293) in patients with COPD. Exacerbation = an increase in symptoms leading to any change in baseline medication or additional medical attention (hospitalization, emergency room visit).
- All patients (including those in placebo group) received rescue albuterol and supplemental ipratropium for use as needed throughout trial, except within 6 hours of PFT visit
BROVANA should not be initiated in patients with acutely deteriorating COPD, which may be a life-threatening condition.