Rescue Medications
37% reduction in daily use of rescue albuterol*1
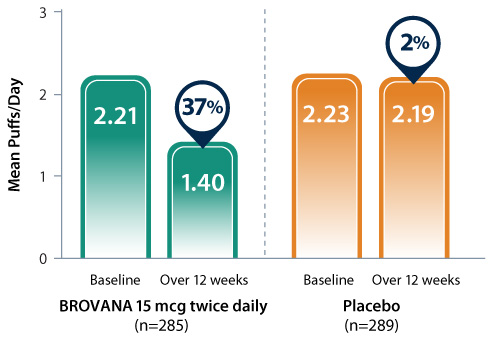
Data from 2 identical, 12-week, double-blind, placebo-controlled, randomized, multicenter trials assessing the efficacy and tolerability of nebulized BROVANA 15 mcg (n=285) vs placebo (n=289) in patients with COPD.2
* Data are pooled from clinical trials A and B over 12 weeks.
37% reduction in daily use of supplemental ipratropium*1
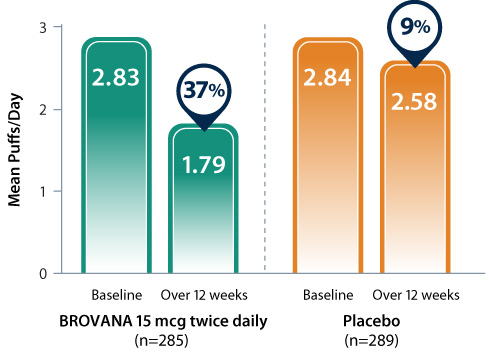
Data from 2 identical, 12-week, double-blind, placebo-controlled, randomized, multicenter trials assessing the efficacy and tolerability of nebulized BROVANA 15 mcg (n=285) vs placebo (n=289) in patients with COPD.20
* Data are pooled from clinical trials A and B over 12 weeks.