Luke
What is trough and why consider it?
- Trough is the low point in a variable measurement1,2
- In COPD, trough FEV1 is the measurement prior to the next dose
Why is trough relevant when treating COPD?
- In COPD, improvement in trough FEV1 of ≥ 100 mL may lead to clinically meaningful outcomes3
- Morning (a time of trough FEV1) is usually when COPD symptoms are more noticeable/at their worst3,4
- 37% of COPD patients reported problems with morning routine as bothersome*5
More frequent dosing intervals can mean more troughs
- Patients using SABA† therapy (≥4 times daily)
- May have 4 or more troughs a day
- GOLD guidelines recommend long-acting bronchodilators for maintenance therapy6
2 doses can mean just 2 troughs
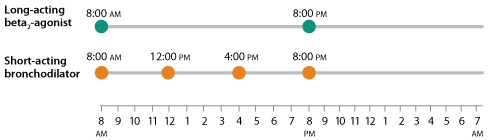
- Inhaled short-acting bronchodilators are recommended as needed for acute symptoms in all stages of COPD
* Quantitative Internet survey of 803 COPD patients, including 289 patients with severe COPD. Questionnaire consisted of 34 questions quantifying the nature, extent, and impact of COPD symptoms at different times of day.
† SABA = short-acting beta2-agonist.
Long-Acting BROVANA may be the right fit
BROVANA: 12-hour bronchodilation, few daily troughs
Significant improvement in mean FEV1, change from baseline over the 12-hour dosing interval at Week 127
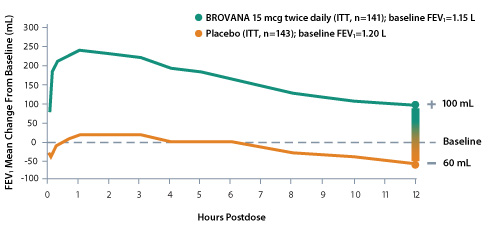
Mean change from baseline in FEV1 at Week 12 in a 12-week, multicenter, randomized, double-blind, placebo-controlled trial to assess the efficacy and tolerability of nebulized BROVANA 15 mcg vs placebo in patients with COPD. Salmeterol was an active comparator, and there was no statistically significant difference between the BROVANA and salmeterol treatment groups for the primary efficacy endpoint.7,8
- In an ad hoc analysis of 212 patients, the majority (56%, n=118) of patients had ≥ 100 mL improvement in trough FEV1 at Week 12*9
- Overall efficacy was maintained throughout the 12-week study period. While some tolerance to the bronchodilator effect was observed after 6 weeks of dosing (at the end of the dosing interval), it was not accompanied by other clinical manifestations of tolerance7,10
- BROVANA improved morning symptom ratings (assessed nighttime symptoms) from baseline†8,11
- BROVANA increased mean symptom-free nights by 19% from baseline over 12 weeks*†12
- All patients (including those in placebo group) received rescue albuterol and supplemental ipratropium for use as needed throughout trial, except within 6 hours of PFT visit
FEV1 = forced expiratory volume in 1 second.
PFT = pulmonary function test.
* Pooled data from 2 identical 12-week, double-blind, placebo-controlled, randomized, multicenter trials (trials A & B) assessing the efficacy and tolerability of nebulized BROVANA 15 mcg twice daily (n=288) vs placebo (n=293) in patients with COPD.
† Patients completed a COPD questionnaire upon rising in the morning to assess nighttime symptoms. Patients reported the number of times they woke during the night due to lung disease symptoms. Symptoms were defined as coughing, wheezing, chest tightness, bringing up mucus, shortness of breath, other.